New Great Britain Biocidal Products Regulation and What it Means for Biocide Products
As of January 1, Great Britain is no longer part of the European Union scheme for regulating biocides. The existing EU Biocidal Products Regulation (EU BPR) was mirrored into Great Britain law as GB Biocidal Products Regulation (GB BPR) and amended to enable it to operate effectively in Great Britain.
What This Means for Biocide Producers
Active substances that were already in the EU Review Program as of December 31, 2020, are now included in the GB Review Program and a GB version of the EU Article 95 list has been established. Stepan Europe is listed as an active substance supplier.
To stay on the GB Article 95 list, an active substance supplier will need to resubmit an application to the Health and Safety Executive (HSE):
- by March 31, when the UK is the evaluating Competent Authority (eCA)
- by June 29, when the UK is not the evaluating Competent Authority (eCA)
To put a biocidal product on the GB market, formulators must be able to demonstrate a clear auditable purchase/supply trail from a GB Article 95 active substance supplier.
Acceptable evidence can include:
- An invoice
- A delivery note
- A letter from a GB Article 95 listed supplier stating that they have a supply agreement with your supplier or that they have supplied the active substance to your company
- If your product requires GB BPR authorization or approval under Control of Pesticides Regulations (COPR), you will need to submit this evidence as part of your application. This evidence must also be supplied to HSE or other Enforcement Authorities, if requested.
Stepan will be pleased to send a letter confirming that Stepan Europe SAS is the active substance supplier in support of our customers.
If interested in marketing a biocidal product in GB, now is the perfect time to reach out to Stepan Europe and speak with one of our European biocidal experts. Click the button below to get in touch with Stepan’s biocide team.
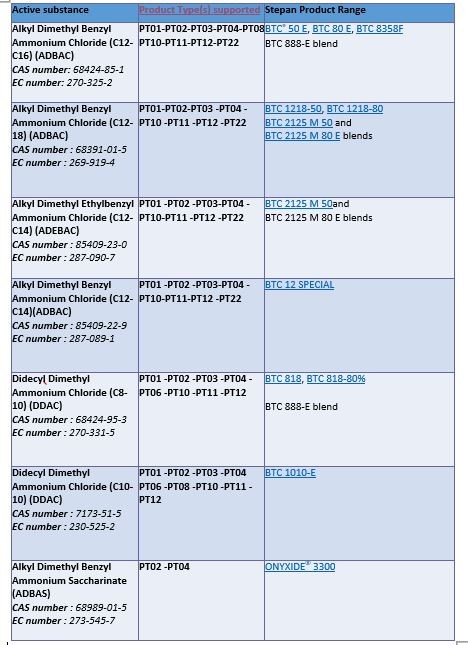
Below is a list of Stepan-supported quaternary active substances and products types.